Chemistry 2021
Objective Questions
The hydrolysis of proteins by diluting mineral acids produces
Correct Answer: C) amino acids
Which of the following oxide causes acid rain?
Correct Answer: D) NO₂
The ratio of carbon atoms to hydrogen atoms in a hydrocarbon is 1:2. If its molecular mass is 56, what is its molecular formula?
Correct Answer: B) C₄H₈
What is the relative molecular mass of the compound below
Correct Answer: B) 136
Cathodic protection of metals is based on
Correct Answer: D) relative tendencies of oxidation
If humid air is polluted by chlorine discharged, the air can be restored by sprinkling
Correct Answer: B) acidified KMnO₄
The alkanol represented by the structure below is
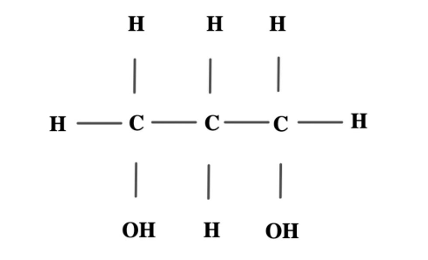
Correct Answer: A) primary and dihydric
Which of the following pairs of compounds would form a precipitate when their aqueous solutions are mixed?
Correct Answer: C) K₂SO₄ and BaCl₂
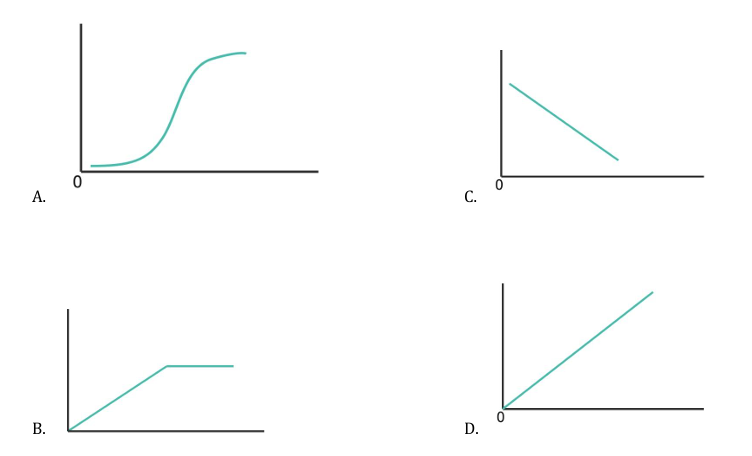
Which of the following illustrates the variation of the rate of evolution of gas from a given length of magnesium ribbon (y-axis) with an increase in the concentration of the added (x-axis)?
Correct Answer: A) I
Which of the graphs illustrates the variation of the pH of a given volume of strong acid solution (y-axis) with the volume of strong base titrated against it (x-axis)?
Correct Answer: A) I
Which of the graphs illustrate the variation of the solubility of a salt in water (y-axis) with increase in temperature (x-axis), if the dissolution process is exothermic?
Correct Answer: C
The formation of ethene from dehydration of ethanol can be described as
Correct Answer:B) an elimination reaction
Which of the following gasses is highly soluble in water at room temperature?
Correct Answer:A) ammonia
A molecule of phosphorus is
Correct Answer:C) tetra atomic
The most common method of preparing insoluble salt is by
Correct Answer:D) double decomposition
What number of moles of oxygen will exert a pressure of 10 atmosphere at 320K in an 8.2dm3 cylinder? [R = 0.082atmdm3/mol/K]
Correct Answer:C) 3.13
The basic property of salt used as drying agent is
Correct Answer:C) hydroscopy
What would be observed when aqueous ammonia is added in drops and then in excess to a solution of copper (II) ions?
Correct Answer:A) blue precipitate is formed which is soluble in excess ammonia
When CuSO4(aq) is added to Pb(NO3)2(aq)
Correct Answer:D) a white precipitate will be formed
Consider the structure below: How many carbon atoms does the parent chain contain?
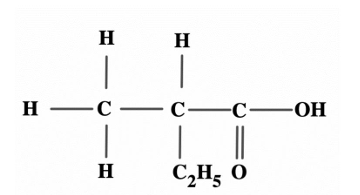
Correct Answer:B) 4
Under which conditions of pressure (P) and temperature (T) would the volume of an inflated balloon increase? When
Correct Answer:C) T is increased and P is decreased
The collision between ideal gas molecules are considered to be perfectly elastic because
Correct Answer:A) they collide without losing energy
Elements with high ionization energy would
Correct Answer:C) have effectively nuclear charges
Which of the following statements about group VII elements is correct?
Correct Answer:C) Their reactivity decreases down the group
Consider the diagram and use it to answer the question below
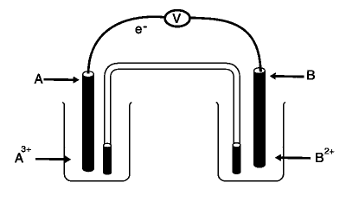
Which of the following cell notations represents the diagram?
Correct Answer:D) A/A3+//B2+/B
Which of the following half-reaction equations represent the reaction at the cathode?
Correct Answer:B) B2+(aq) + 2e– → B(s)
The reactivity of fluorine is high because of
Correct Answer:A) its high electronegativity
How many coulombs of electricity would liberate 1.08g of Ag from a solution of silver salt? [Ag = 108.0; F = 96500 C]
Correct Answer:C) 965 C
The Bohr model of the atom proposed the existence of
Correct Answer:B) electron shells
At 25°C evaporation of a 100cm3 solution of K2CO3 to dryness gave 14g of the salt. What is the solubility of K2CO3 at 25°C? [K2CO3 = 138]
Correct Answer:C) 1.01 mol/dm3
Student X titrated 25cm3 of Na2CO3 with 0.1 mol/dm3 HCl using methyl orange as indicator. Student Y carried out the same exercise but he used phenolphthalein as indicator. Which of the following statements about the titration is true?
Correct Answer:C) The titre value obtained by X is twice that of Y
What is the concentration of a solution which contains 0.28g of KOH in 100cm3 of solution? [KOH= 56]
Correct Answer:B) 0.05 mol/dm3
What is the empirical formula of a hydrocarbon containing 0.160mol of carbon and 0.640 moles of hydrogen?
Correct Answer:B) CH3
Which of the following species has the largest ionic radius?
Correct Answer:A) S2-
Which of the following statements is correct about ionization energy?
Correct Answer:D) Decreases down the group
Potassium trioxonitrate (V) can be obtained from its solution by
Correct Answer:C) Crystallization
An element, Q, contains 69% of 63Q and 31% of 65Q. What is the relative atomic mass of Q?
Correct Answer:B) 63.6
The following ions have the same electron configuration except 8O, 12Mg, 13Al, 17Cl.
Correct Answer:A) Cl–
The region around the nucleus where electrons can be located is called
Correct Answer:B) an orbital
Protons and electrons are fundamentals particles because they
Correct Answer:D) are found in all matter
Consider the following energy profile diagram and use it to answer the question that follows.
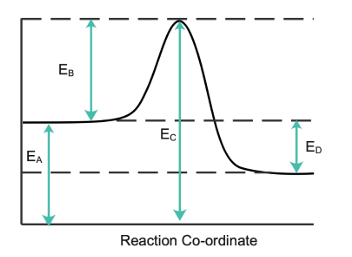
The activation energy of the reaction is
Correct Answer:B) EB
The enthalpy change of the reaction is
Correct Answer:D) ED
The energy profile diagram illustrates
Correct Answer:B) an exothermic reaction
How many molecules of oxygen would occupy a volume of 2.24 cm3 at S.t.p.?
Correct Answer:C) 6.02 x 1019
The isotopes of neon are represented by the symbols 20xNe, 21yNe and 22zNe. The relationship between x, y and z is
Correct Answer:C) x = y = z
Which of the following pairs of molecules form hydrogen bond?
Correct Answer:A) C2H5OH and CH3OH
Which of the following statements about elements in group VII is correct?
Correct Answer:D) I2 is a stronger oxidizing agent than F2
Electrovalent compounds normally
Correct Answer:D) dissolve in polar solvents
A coordinate covalent bond could be formed between
Correct Answer:D) H+ and AlCl3
Which of the following scientists discovered the electron?
Correct Answer:A) Joseph J. Thompson
Theory Questions
-
(a) Distinguish between molecular formula and structural formula.
(b) List three factors that determine the ionization energy of an atom.
(c) State two conditions necessary for the establishment of chemical equilibrium.
(d) Consider the following table:
Element A B C Ionization energy (kJ/mol) 619 518 594 (i) State which of the elements is the strongest reducing agent.
(ii) Give a reason for your answer.
(e) State Graham’s law of diffusion.
(f) Consider the following salts:
- Mg(NO3)2
- CaCO3
- Na2SO4
State which of the salts is/are:
(i) readily soluble in water
(ii) insoluble in water
(g) Classify each of the following as addition or condensation polymers:
- Protein
- Perspex
- Nylon
(h) Define atomic radius.
(i) Explain briefly why ethanol has a higher boiling point than propane although they have comparable molar masses.
(j) State three significances of pH value in everyday life.
-
(a)
(i) State two characteristics of a homologous series.
(ii) Explain briefly why ethane and ethene differ in their reactions.
(b) When crystals of sodium chloride were warmed with concentrated tetraoxosulphate(VI) acid:
(i) Name the gas evolved.
(ii) State two properties of the gas.
(iii) Write a balanced chemical equation for the reaction.
(c)
(i) What are hydrocarbons?
(ii) State two natural sources of hydrocarbons.
(iii) A hydrocarbon contains 83% carbon by mass. Calculate its empirical formula.
(d) Draw and label a diagram of a setup used to electroplate a copper ornament with silver.
-
(a) In an experiment, 25.0 cm3 of H2SO4 neutralized 24.0 cm3 of 0.150 mol dm-3 KOH.
(i) Write the balanced equation.
(ii) Calculate the concentration of the acid.
(b)
(i) A burning magnesium ribbon was placed in carbon (IV) oxide.
(I) Write the equation.
(II) Explain why magnesium burns in CO2.
(ii) Calculate the percentage by mass of nitrogen in magnesium trioxonitrate(V).
(c) Given CH3CH2CH=CHCOOH:
(i) State two chemical tests.
(ii) State the observations.
(d) Describe briefly how soap is manufactured.
(e) Define electronegativity.
-
(a)
(i) Describe the structure of graphite and diamond.
(ii) Explain their differences in hardness and conductivity.
(b)
(i) State what is achieved during aeration and sedimentation in water purification.
(ii) Name two substances that cause hardness in water.
(iii) State two methods of removing hardness.
(iv) Give one disadvantage of hard water.
(c)
(i) Describe briefly the extraction of tin.
(ii) Write equations involving oxygen and chlorine.
-
(a)
(i) Explain why aluminium is not affected by air.
(ii) In aluminium extraction, state:
(I) Substance used for purifying bauxite
(II) Composition of the electrolyte
(b) Show that ZnO is amphoteric using equations.
(c)
(i) List three uses of sodium trioxocarbonate(IV).
(ii) Explain why trioxonitrate(V) acid turns yellow on storage.
(iii) Describe a laboratory test for trioxonitrate(V) ions.
(d)
(i) Write equations for the preparation of hydrogen chloride.
(ii) State one use of hydrogen chloride.